Mass Density Of Water In Kg M3
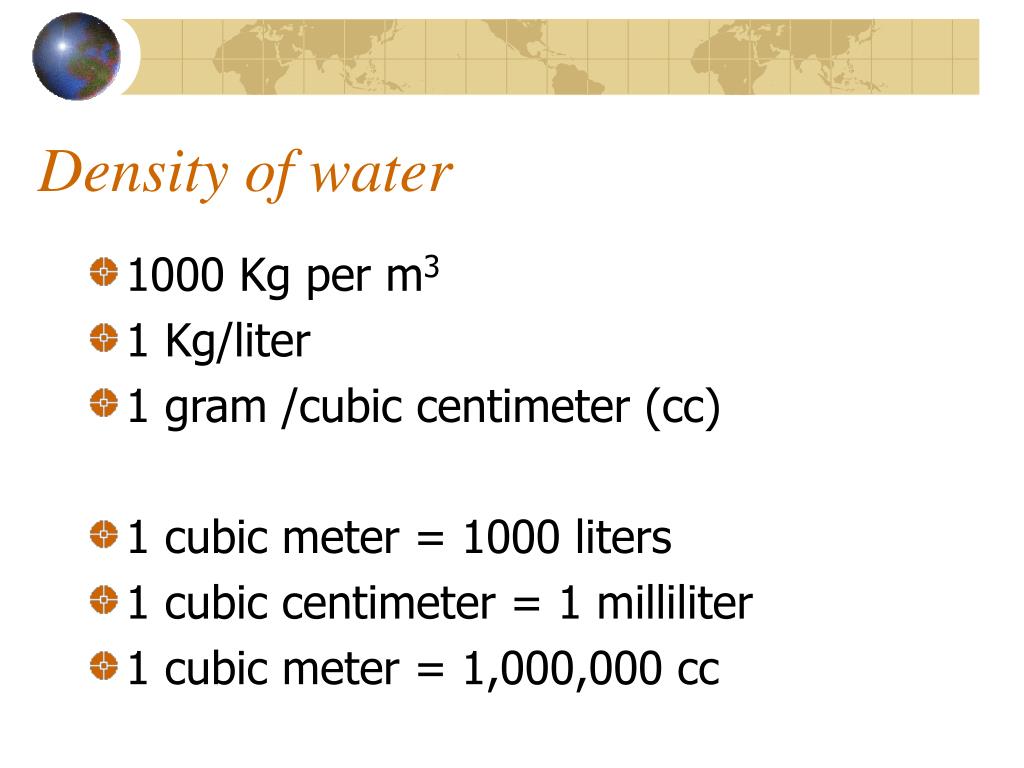
Let's talk about something seriously weighty. No, not your uncle's questionable holiday fruitcake. We're diving into the surprisingly fascinating world of water density. Specifically, the number that pops up in science class and makes you think, "Is that really a thing?" I'm talking about 1000 kg/m³. Yes, a thousand kilograms for every cubic meter of water. Sounds precise, right? Almost… too precise.
Now, I have a confession. I’ve always been a little suspicious of this number. It feels like water got together with a bunch of other stuff and decided to gang up on us with this neat, tidy figure. Like, "Oh, we're water? Let's just all agree to be exactly 1000 kg/m³. Sounds good? Great!" It feels… manufactured. Like the universe just needed a nice, round number to fill in a blank on a cosmic spreadsheet.
Think about it. What is a cubic meter? It’s a big cube, like a small refrigerator. Imagine filling that entire fridge with water. And then, poof, it magically weighs as much as a thousand kilograms. That’s like loading up a compact car with about 2200 pounds of groceries. It’s a lot of stuff. And water, this seemingly simple liquid, is just… chilling there, being that heavy. How does it do it?
My personal theory? It’s a collective agreement. The H₂O molecules are having a huge, silent meeting, perhaps somewhere deep in the Mariana Trench. "Alright everyone, huddle up!" says a particularly enthusiastic oxygen atom. "We need to hit this 1000 kg/m³ target. Let's all get cozy. No slacking!" And they all squeeze together, forming this perfectly dense, uniformly heavy substance. It’s like a very orderly, very wet flash mob.
And have you ever noticed how persistent this number is? You can boil it, freeze it, even turn it into steam (though I suspect steam is cheating, like the molecules are taking a brief vacation from their density duties). But the moment it’s back to being plain old liquid water, there it is again. 1000 kg/m³. It's like a stubborn houseguest who just refuses to leave, no matter how many hints you drop.

I sometimes wonder if the scientists who first measured this were just really, really good at guessing. Like, "Hmm, feels about right. Let's call it 1000. That's a nice, solid number." And then it stuck. For centuries. Imagine the pressure! Being the person who has to verify that water is still 1000 kg/m³. "Yep. Still there. No change. Moving on." The sheer monotony must be mind-numbing.
And the fact that it’s in kilograms per cubic meter? It sounds so official. So scientific. So… unyielding. It's not just "heavy." Oh no. It's kilograms. And it's a whole meter cubed of it. It’s like water put on its best suit and tie to impress us with its mathematical prowess. "Look at me," it whispers, "I'm not just wet; I'm quantifiable."
I’ve tried, you know. I’ve stared into my water glass, trying to feel the 1000 kg/m³. Does it have a certain gravitas? A weighty presence? Or is it just… wet? My untrained senses tell me it's mostly just wet. But then I remember the number. The infamous 1000 kg/m³. And I get this strange sense of respect. Or maybe mild bewilderment.
It's this little secret that water keeps. This precise, imposing measurement that it doesn’t exactly advertise. You don’t see it on the label of a bottled water: "Contains 1000 kg/m³ of pure hydration!" No, it’s hidden away in textbooks, waiting to trip up unsuspecting students. It’s the scientific equivalent of a hidden Easter egg, except this egg is incredibly heavy and you can’t eat it.
Perhaps my unpopular opinion is that water is a bit of a show-off. It presents itself as simple, clear, and essential. And it is! But beneath that placid surface, it’s secretly harboring this impressive, precisely defined density. It’s the quiet achiever of the liquid world. The one who gets A's on every test without breaking a sweat… or, in this case, without altering its density by even a fraction.
So next time you’re drinking water, or taking a bath, or just staring blankly at a puddle, take a moment. Contemplate the 1000 kg/m³. Marvel at the collective effort of all those H₂O molecules, diligently maintaining their posts. It’s a small, but mighty, testament to the universe’s love for a good, solid number. And perhaps, just a little bit, to water’s own secret, weighty ambition.
