At What Temperature Does The Ice Melt
Hey there, fellow ice enthusiasts! Ever looked at a perfectly formed ice cube, or maybe a glistening icicle, and wondered, "When exactly are you gonna take the plunge and become a puddle?" It's a question that pops into our heads, especially when we're waiting for that chilled drink or contemplating the mysteries of winter. Well, buckle up, because we're about to dive deep (but not too deep, we're talking about water here!) into the fascinating world of melting ice.
So, what's the magic number? The temperature at which ice decides it's had enough of being solid and wants to join the liquid party? Drumroll, please... it's 0 degrees Celsius, or for all you folks who love Fahrenheit (and let's be honest, it's pretty handy for everyday stuff), that's a cozy 32 degrees Fahrenheit.
Yep, that's it! That's the big reveal. 0°C or 32°F. Pretty straightforward, right? It's like the universe's universal "on" switch for becoming a liquid. But wait, don't go scrolling away just yet! This isn't just a simple number; it's the gateway to a whole bunch of cool science (pun intended!).
The Sciencey Bit (Don't Worry, It's Fun!)
So, why 0°C? It all comes down to how water molecules like to hang out. When water gets really, really cold, those molecules get a bit sluggish. They slow down and start to arrange themselves into a super organized, rigid structure. This structure is what we call ice. Think of it like a perfectly choreographed dance where everyone knows their spot and stays put. Very polite, very structured.
But, when you start warming things up, those molecules get a jolt of energy. They start wiggling and jiggling more. They can't hold their rigid positions anymore. It's like the music suddenly sped up, and the dancers are like, "Whoa, this is too much effort to stay in formation!"
When the temperature hits that sweet spot of 0°C (or 32°F), it's like the molecules are right on the edge of chaos. They have just enough energy to break free from their icy bonds and start sliding past each other. Voila! You've got liquid water. It's a bit more free-spirited, a bit more spontaneous. Less of a ballroom waltz, more of a free-for-all dance party.

What About Different Kinds of Ice?
Now, you might be thinking, "But what about the ice in my freezer? It seems pretty solid, even if it's at 0°C!" Ah, excellent question, my sharp-witted friend! Your freezer is usually set colder than 0°C, often around -18°C (0°F). This is to keep things really frozen and prevent any unwanted thawing or bacterial growth. It's like tucking your ice cubes into a super-duper cold sleeping bag to make sure they stay frosty.
And what about that frosty layer on the outside of your ice cream tub? That's also ice, and it melts at the same magical temperature. The only difference is the surrounding environment. If the air around the ice is below 0°C, it stays frozen. If it creeps up to or above 0°C, even if the inside of your freezer is colder, the surface will start to melt.
Think about it like this: If you have a perfectly built snowman in your yard when it's -5°C (23°F), he's going to be a happy, frosty fellow. But if the temperature outside climbs to 1°C (34°F) and your snowman is still there, he's going to start looking a little sad and melty. The snowman itself hasn't changed its fundamental melting point, but its environment has.
Beyond the Simple Number: Factors That Can Play a Role
While 0°C is the magic number for pure water ice, life isn't always pure, is it? Sometimes, other things can get mixed in, and this can actually affect the melting point. It's a bit like adding a sprinkle of fairy dust, but with scientific consequences!
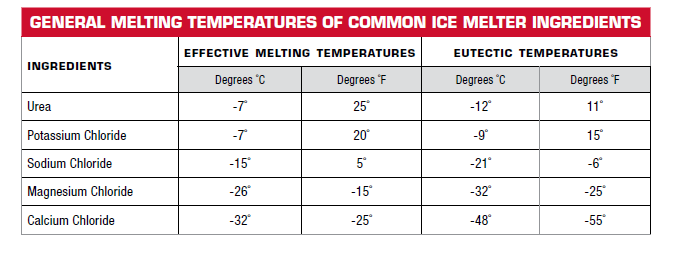
Salt is the most common culprit. Ever noticed that roads are salted in winter? That's not just for fun (though it does make driving a bit less slippery, which is kind of fun). Salt dissolves in water and lowers the freezing point. So, that salty slush on the road isn't just melted snow; it's a mixture that stays liquid even below 0°C. This is why you can have puddles of salty water when the air temperature is still quite chilly.
How does this happen? Well, those salt ions (the little charged bits of salt) get in between the water molecules. They disrupt the orderly dance of the water molecules trying to form ice. It takes even less energy (i.e., a lower temperature) for the water molecules to break free when those salty interlopers are around.
So, if you have ice with salt mixed in, it will melt at a temperature below 0°C. The more salt you add, the lower the melting point goes. Pretty neat, huh? It’s like giving the ice molecules a little nudge to get them moving sooner.
Other dissolved substances can have similar effects, though salt is the most dramatic and commonly encountered in our daily lives. Sugar, for example, also lowers the freezing point, though not as much as salt. This is why if you add sugar to your iced tea, the ice might melt a tiny bit faster, but it's usually not a noticeable difference unless you're doing a science experiment.

The Pressure Thingy
Okay, here's a slightly more obscure fact that might blow your mind a little. Pressure can also affect the melting point of ice. Now, for the most part, the pressure changes we experience in our daily lives (like going up a mountain) have a very small effect on ice melting. We're talking tiny, microscopic changes.
However, under extreme pressure, the melting point can change more significantly. For instance, incredibly high pressures can actually increase the melting point of ice, meaning it would need to be warmer than 0°C to melt. This is a fascinating phenomenon that happens deep within the Earth or in the extreme conditions found on other planets.
But don't worry about your ice cubes in your drink suddenly refusing to melt because you're sitting on a slightly uneven chair. The pressure change is so minuscule, it's practically non-existent in our everyday world. So, while it's scientifically cool, it's not something you need to stress about when you're just trying to enjoy a refreshing beverage.
The Importance of the Melting Point
So, why do we care about this 0°C (or 32°F) number? Well, it's a fundamental property of water, and water is pretty darn important to, you know, life. Understanding the melting point of ice is crucial for so many things:

- Weather Forecasting: Meteorologists use this to predict when snow will turn to rain, when ice will form on roads, and how quickly snow will melt. This helps keep us safe and plan our days.
- Food Preservation: Refrigerators and freezers rely on keeping things below 0°C to prevent ice from melting and food from spoiling.
- Agriculture: Farmers need to know when frost might occur (freezing) and when it will thaw to protect their crops.
- Engineering and Construction: Building bridges, roads, and buildings in cold climates requires an understanding of how ice forms and melts, and the stresses it can put on structures.
- Everyday Life: From making ice cream to keeping your drinks cold, the melting point of ice is a constant, silent player in our daily routines.
It's amazing how a single, seemingly simple number can have such a profound impact on our world. It's the invisible line that separates a solid, frozen landscape from a flowing, liquid one. It’s the point where winter gives way to spring, or where a refreshing drink gets its chill.
The Joy of Melting
Think about it for a second. When that ice cube in your glass starts to "sweat," and then eventually melts into that cool, refreshing water, it's a small, everyday miracle. It's a reminder that change is constant, and that sometimes, the most beautiful transformations happen when things warm up.
That slow drip from an icicle is nature's own little countdown to spring. The way ice sculptures transform from magnificent art pieces into glistening puddles is a poignant, albeit temporary, display of the passage of time. And honestly, the satisfaction of seeing a perfectly chilled drink get even more diluted and refreshing as the ice melts? Priceless.
So, the next time you see ice melting, whether it's in your drink, on the street, or forming a beautiful shape in nature, take a moment to appreciate it. It's more than just water turning liquid; it's a fundamental process that shapes our world, allows for life, and brings us those delightful moments of refreshment and change. Keep an eye on that temperature, and embrace the melt!
